Tissue Manual | Collecting, Handling, & Storing Tissues
Tissue Collection and Transport
Protocols for the collection of tissues vary depending on the situation and preservation method. Review the “Guidelines for Collection of Bird Tissues” for protocols specific to bird tissues.
The most common methods for preserving tissues in the field are frozen (liquid nitrogen), RNALater, or ≥95% ethanol. The method of preservation depends on a number of factors, including the goals of the study, logistics of transport, and permit restrictions. Some studies warrant preservation of multiple tissue types in different vials for genomic analysis. Imports from countries that are listed as having highly pathogenic avian influenza must be preserved and treated in specific ways prior to import, as authorized on the museum’s USDA import permit. Bats and other organisms that pose health hazards to animals or people also may require special treatment. It is important to know the regulations and discuss preservation methods with a Curator in advance of planning fieldwork.
Relevant Links
The MVZ provides vials, barcodes, pens, and other supplies for collection of tissues that will be deposited into its collections. RNALater is available through the MVZ genetics lab or tissue facility. The museum also has a limited number of nitrogen tanks and dry shippers of various sizes that may be borrowed upon request. These typically belong to individual curators, who should be consulted prior to checking out a tank. Contact a Staff Curator for basic supplies, and Terri Barclay for access to the nitrogen tanks. There are also two portable –20 C freezers that may be available to loan upon request.
Tissues should be put into 2 mL sterile cryogenic Nalgene vials to enable easy installation into the collection (purchased in bulk, 1000 vials per case). Barcodes should be put on all vials to be deposited in the MVZ.
Download Guidelines for Bird Tissue Samples (PDF). This document includes guidelines for preserving bird tissues in liquid nitrogen, RNALater, or buffer/ethanol, as well as preservation of blood on microscope slides.
If tissue is saved for a specimen, this should be indicated in the field catalog along with other data. The number of vials per specimen (if more than 1) and the type of tissue preservation also should be noted (e.g., “frozen tissue” and/or “tissue in 95% EtOH”). If the preservation method is not indicated, the default is assumed to be frozen. Standard tissues collected are heart, liver, muscle, and/or kidney (often noted as H, L, M, K). If multiple vials are saved for a specimen, that should be noted in the field catalog along with the tissue type(s) in each vial (e.g., vial 1=H,L; vial 2=M,K). Non-standard tissue types also should be noted (e.g., “tail tip,” “toe clip,” “scale,” “blood,” etc.).
Data for samples that are non-vouchered (either because a voucher is not saved or because it is left elsewhere for disposition) should be recorded in a field catalog in the same manner as vouchered material. Non-vouchered samples should be given a field number in sequence with other material as if a traditional specimen were saved and cataloged by the collector, and all relevant data (locality, date, ecological or behavioral information, etc.) should be noted. Disposition of the voucher also should be recorded (e.g., “specimen discarded,” “animal released,” “voucher left in Mexico at UNAM,” etc.). Again, it is important to note tissue type in the field catalog.
Animals that are brought to the Museum alive for later preparation should be given a collector number in sequence with other material, with the annotation that the “specimen” is alive. Data on specimen preparation and tissue preservation should be added at the time that the animal is killed and preserved. However, if a piece of tissue (e.g., a tail tip) is saved when the animal is initially collected, that should be noted in the field catalog.
IMPORTANT NOTE: A lot of work goes into collecting and preparing specimens, and it is critical to be as clear as possible when recording the data. Keep in mind that undergraduates do most of the cataloging, data entry, and tissue scanning, and rely on the data obtained from field/prep catalogs. If the writing is hard to read, hidden by the barcode, or otherwise difficult to interpret, it makes their job more difficult and reduces the quality of the data.
Individual researchers are responsible for obtaining information on rules and regulations for international transport of tissues and associated equipment/supplies on commercial airlines. However, some guidelines are provided here.
Liquid nitrogen is prohibited, but dry shippers are allowed on commercial airlines. However, the pilot and other airline staff have the authority to decline transport of a tank at their discretion. If you are planning to fly commercially after completing a field trip during which frozen tissues have been collected, we recommend the following procedures:
- empty the tank right before departure
- pour some of the LN2 over “blue ice” to freeze them at ultracold temperatures
- put the tissues along with frozen “blue ice” in a soft-sided, insulated cooler (such coolers may be eligible for “carry-on” baggage status, depending upon size restrictions)
Alternatively, the tissues can remain in the emptied tank. If the tank is in good condition, the tissues should remain frozen for at least 24 hours. One tip is to add vials half-filled with water into the tank before the nitrogen is emptied; these act as ultracold “ice cubes.” Note that a tank may fail at any time for unknown reasons, so having a second “back-up” tissue vial in buffer or ethanol provides insurance against potential loss.
Both the empty tank and cooler can be transported as checked baggage. However, it is a good idea to check with the airlines to make sure that they do not have any special regulations. It is also a good idea to declare the tank, stating simply that it is an empty cooler for scientific samples.
Ethanol is considered a dangerous substance in certain quantities and concentrations; thus, shipping and/or transportation are regulated. Vials with ethanol should be well-sealed to prevent leaking (e.g., with parafilm, in a plastic bag or box). Ethanol- or buffer-preserved tissues should be packed with checked baggage and not hand-carried.
See Permits for information about permits relating to the importation of tissues.
Labeling Tissue Vials
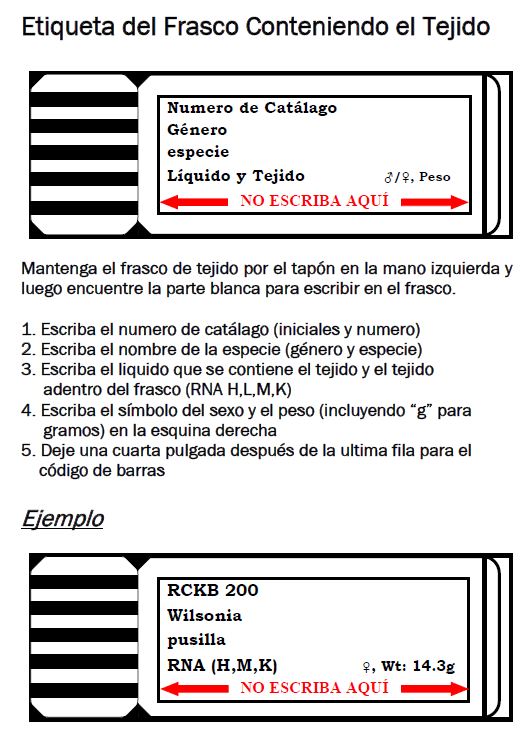
All tissue vials should be labeled with the collector’s or preparator’s initials and field/prep number, and with the taxonomic identification of the specimen. Specimens that cannot be identified fully should be labeled with the best possible identification (e.g., generic name, family, etc.). Additional information that’s useful to put on the vial includes sex and weight (the latter to help troubleshoot if there are numbering issues). If multiple vials are saved, then it is also important to write the tissue type(s) on each vial so that information can be added to the MVZ database; tissue types may be abbreviated with one letter (e.g., H=heart, L=liver, M=muscle, K=kidney, B=brain, E=eye, G=gonad).
Vials should be labeled with Cancer Diagnostic pens (available from a Staff Curator) on the white area with the cap pointing to the left. It is important to leave at least 3 mm at the bottom of the white area without writing because of overlap with the barcode; if there is writing below that area, it will be hidden by the barcode.
The brief video below shows how to label and barcode tissue vials for the MVZ collections. It also provides some guidance on the types and order of tissues preserved (example is for birds; the types of tissues preserved may differ among collections).
Handling Tissues on Dry Ice
When working with frozen tissues in the MVZ liquid nitrogen facility, samples should be kept on dry ice at all times to keep them frozen. Dry ice is available for use on a recharge basis. The dry ice cooler is located between the VLSB and LSA doors on the first floor, behind a door that goes to the loading dock and dumpsters. When taking dry ice, record the number of scoops on the clipboard sheet with your name and “MVZ Curatorial” as the lab. There is also a gray dry ice cooler in that room for storing dry ice that is left over.
Dry ice is delivered once or twice a week. If the dry ice bin is empty or low, please notify the person working in the LSA stockroom. If you need to work and there is no dry ice, you can use frozen ice packs (preferably ultracold-frozen) as a temporary solution until dry ice is available again.
Storing Uncataloged Tissues
The museum provides space for storing uncataloged frozen material, either in an ultra-low freezer on the fourth floor or in one of the liquid nitrogen tanks on the first floor. Samples that are being actively accessed for lab work (including field-collected samples and tissue loans) should be stored on the fourth floor. Access to the liquid nitrogen tanks is restricted, and samples should be given to a Staff Curator who will then put them in liquid nitrogen storage. There also is a chest ultra-low freezer on the first floor in the liquid nitrogen facility, but this is intended for samples that are in the process of curation. Use the white board near each chest freezer to indicate the location of boxes within that freezer.
All samples should be stored in 9 x 9 plastic/polycarbonate boxes that fit in the racks. Do not use cardboard boxes if possible. Boxes are available from a Staff Curator, and they should be used ONLY for freezer storage within the MVZ. They are not to be taken when people (e.g., grad students, postdocs, etc.) leave. Each box should be labeled with the person’s name and nature of material (e.g., “Conroy, San Bernardino Mts., April 2002” or “gift from Lassen National Park, 2002”). Boxes that are stored in a liquid nitrogen tank must be scanned into Arctos so that the location can be tracked.
Never store tissues in loose bags or containers that don’t fit in the racks, and never store tissues on top of the racks in the freezer!
Each collection has its own system for tracking and curating uncataloged tissues. For tissue curation protocols, see Tracking Uncataloged Tissues.
TISSUES PRESERVED ON BEHALF OF ANOTHER MVZ RESEARCHER: Occasionally, someone will preserve tissues on behalf of another researcher. Tissues that are assigned that researcher’s collector number should be put either in an appropriate box for cataloging, or in a box with other tissues collected by that researcher. If such tissue is part of an accession that has not yet been cataloged, it should be placed with the other material of the same accession. Such samples should not be mixed with tissues from the person who did the preservation.